


BioSieve™ Fentanyl FIA Home Test Kit and BioSieve™ Toxismart FIA Reader Get FDA Approval for OTC Use
We are proud to announce that VivaChek a leading innovator in diagnostic solutions, has achieved a significant milestone. Our BioSieve™ Fentanyl FIA Home Test Kit and BioSieve™ Toxismart FIA Reader have received FDA approval for over-the-counter (OTC) use, making them the first-ever devices of their kind to reach this milestone.

VivaChek to Present Latest Advancements at Medica 2024 in Düsseldorf
VivaChek announces its participation in Medica 2024, the world’s largest medical trade fair, taking place in Düsseldorf, Germany, from November 11–14, 2024. We will be showcasing our latest developments in Multifunction Monitoring Systems and point-of-care testing (POCT) solutions at Booth No. Hall 3 K52.

VivaChek Opens New Headquarter Campus, Marking a Milestone in Company Growth
On October 10, 2024, VivaChek celebrated a significant milestone with the official inauguration of its new headquarters campus located at 17 Wuzhou Road, Linping District, Hangzhou China. This marks an important step in the company's continuous growth and development within the in vitro diagnostics industry.

VivaChek Biotech Achieves FDA Clearance for Three Innovative Glucose Meters
We are excited to announce that VivaChek Biotech has received FDA clearance for three of its glucose meters featuring advanced FAD enzyme technology. This key milestone underscores the company’s steadfast commitment to driving innovation and maintaining the highest quality standards in healthcare.

VivaChek Achieves 510(k) Clearance for BioSieve™ Fentanyl FIA Test Kit and BioSieve™ Toxismart FIA Reader
We are excited to announce that VivaChek has achieved a significant milestone with the US FDA 510(k) clearance of our BioSieve™ Fentanyl FIA Test Kit and BioSieve™ Toxismart FIA Reader. This groundbreaking approval marks the first of its kind in the United States, setting a new standard in fentanyl detection technology.
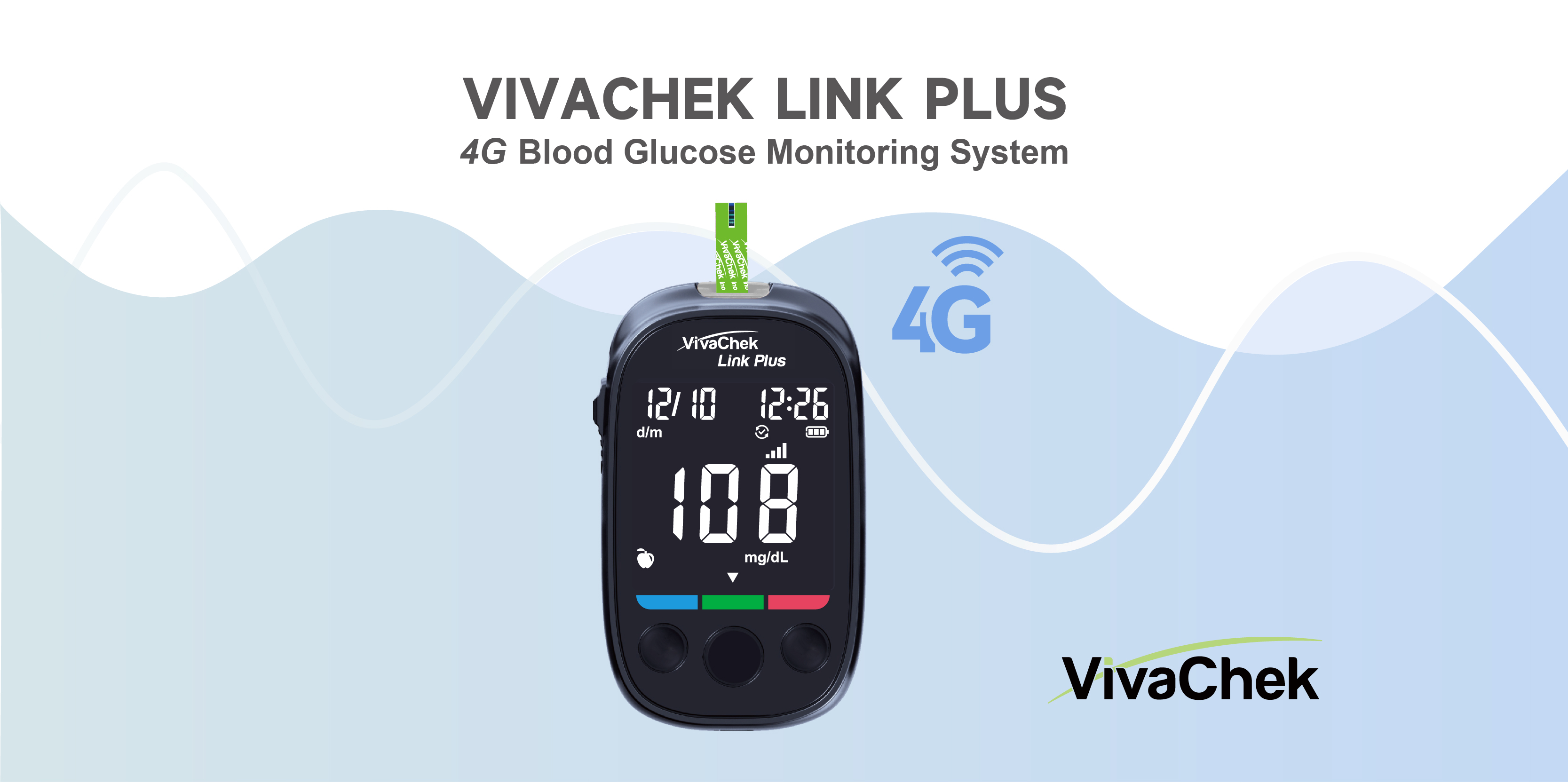
VivaChek received FDA 510(K)clearance for VivaChek Link Plus 4G Blood Glucose Monitoring System
We are excited to announce a significant milestone for VivaChek: our revolutionary VivaChek Link Plus Blood Glucose Monitoring System received FDA 510(k) clearance on December 22th. This achievement marks a substantial advancement in diabetes management technology, signifying a noteworthy leap forward in our commitment to innovation and healthcare excellence.

VivaChek to Attend FIME 2024
VivaChek is excited to announce our participation in the upcoming FIME 2024 exhibition. Join us at booth No: K37, where we will be showcasing our latest innovations, including the 4G glucose meter, Multi-Drug Test penal and newly launched VivaChek Lactate Analyzer.

Successful launch of BioSieve DOA products at NDASA
The NDASA 2023 Conference & Trade Show sponsored by National Drug & Alcohol Screening Association will take place in Bellevue, Washington, from May 23 to May 25, 2023.The NDASA advocates for safe and drug-free workplaces and communities through legislative advocacy, education, training and excellence in drug and alcohol screening services.

Great News - VivaChek Receives FDA 510(k) Clearance For Multi-Drug Urine Test Cup
VivaChek received FDA 510(k) clearance for its Multi-Drug Urine Test Cup which aims to provide an accurate and less invasive way for drug testing. A 510(k) is a premarketing submission made to US. Food and Drug Administration and the clearance demonstrates that the device to be marketed is as safe and effective.
